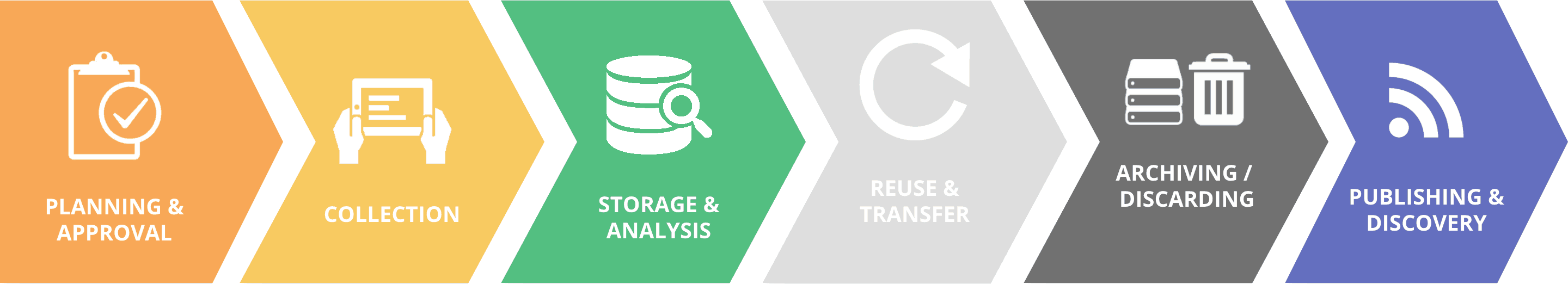
Responsible Data Guidelines – Additional resources
International Ethical Guidelines for Health-related Research Involving Humans
By Council for International Organizations of Medical Sciences (CIOMS) | 2016
Prepared by the Council for International Organizations of Medical Sciences (CIOMS) in collaboration with the World Health Organization (WHO). The ethical principles set forth in these Guidelines should be upheld in the ethical review of research protocols. The ethical principles are regarded as universal.
This document is a suggested additional resource in our Responsible Data Guidelines.