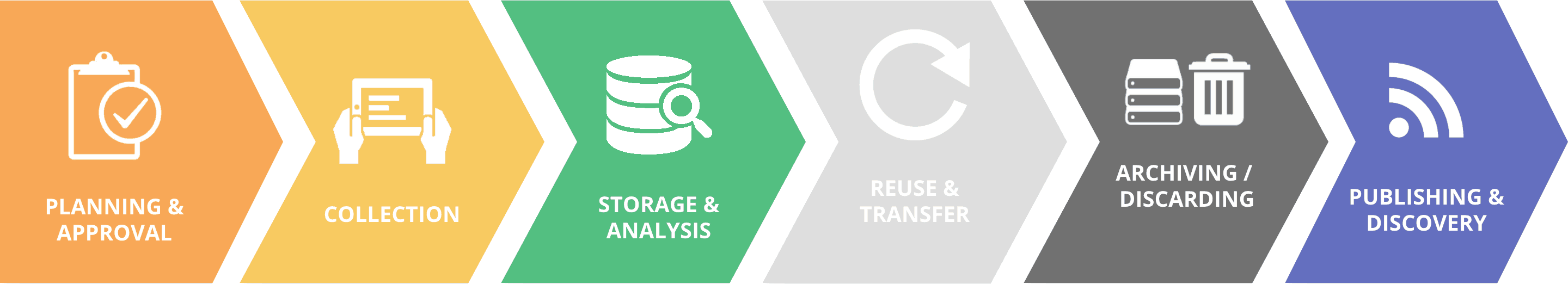
Responsible Data Guidelines – Additional resources
Pseudonymization of patient identifiers for translational research
By BMC Medical Informatics and Decision Making | 2013
The usage of patient data for research poses risks concerning the patients’ privacy and informational self-determination. But if biospecimen from patients are anonymized, individual research results cannot be associated back to the individual and cannot be offered to patients in a clinically relevant timeframe. This paper presents a new approach which supports both data privacy and the possibility to give feedback to patients about their individual research results.
This document is a suggested additional resource in our Responsible Data Guidelines.