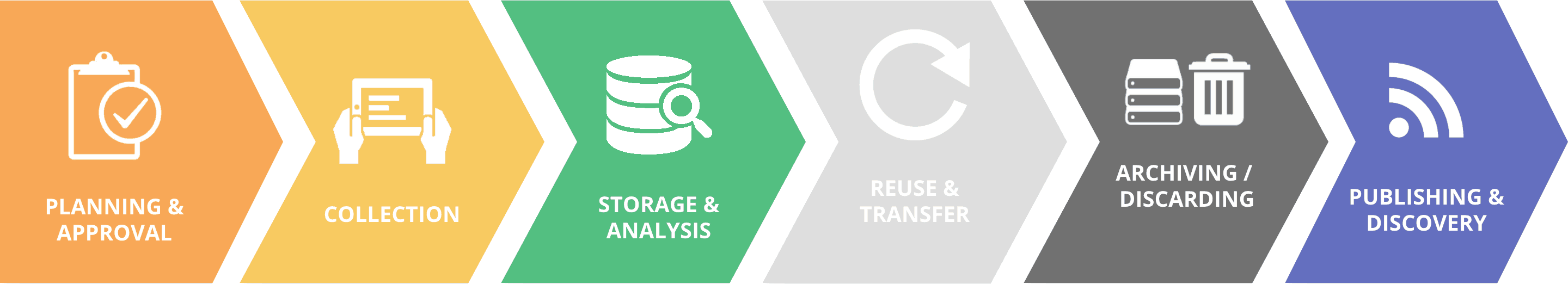
Responsible Data Guidelines – Additional resources
Standards and Operational Guidance for Ethics Review of Health-Related Research with Human Participants
By World Health Organization (WHO) | 2011
This document has been developed for individuals and organizations involved in health-related research with human participants. In particular, this document is intended to provide guidance to the research ethics committees (RECs) on which organizations rely to review and oversee the ethical aspects of research, as well as to the researchers who design and carry out health research studies.
This document is a suggested additional resource in our Responsible Data Guidelines.