
2020 Convention session – Data Sharing in a Digital World
This session on data sharing in a digital world aired live at the 2020 virtual CGIAR Convention on Big Data in Agriculture.

by Hannah Craig | Nov 24, 2020 | Video
This session on data sharing in a digital world aired live at the 2020 virtual CGIAR Convention on Big Data in Agriculture.
by Hannah Craig | Nov 24, 2020 | Video
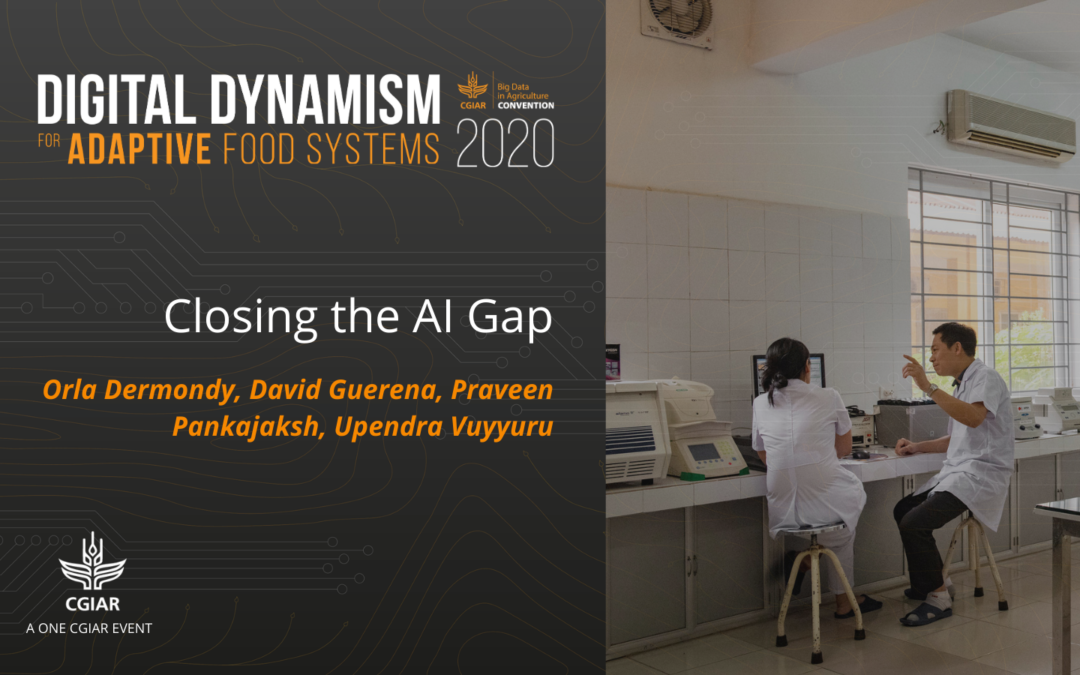
This session on closing the AI gap aired live at the 2020 virtual CGIAR Convention on Big Data in Agriculture.

by Hannah Craig | Nov 23, 2020 | Video
This session on the CGIAR digital strategy aired live at the 2020 virtual CGIAR Convention on Big Data in Agriculture.
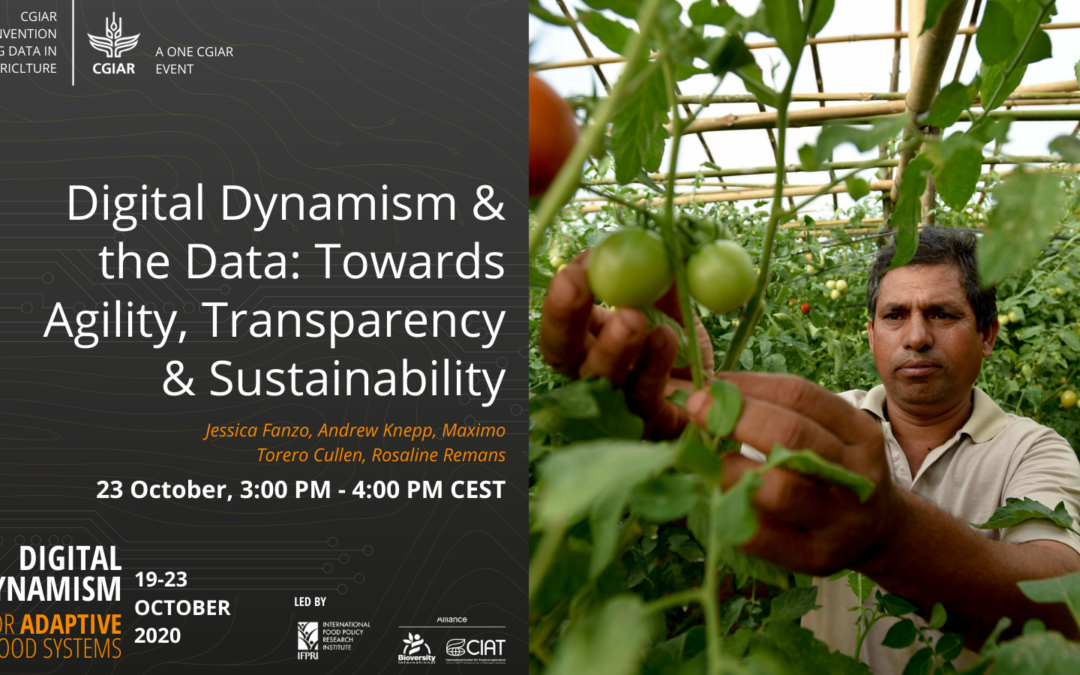
by Hannah Craig | Nov 23, 2020 | Video
This session on big data to scale aired live at the 2020 virtual CGIAR Convention on Big Data in Agriculture.

by Hannah Craig | Nov 23, 2020 | Agronomy CoP, Video
This session on big data to scale aired live at the 2020 virtual CGIAR Convention on Big Data in Agriculture.
CGIAR Platform for Big Data in Agriculture advocates open data for agricultural research for development. It considers that opening up research data for scrutiny and reuse confers significant benefits to society.
However, the Platform appreciates that not all research data can be open and that a broad range of legitimate circumstances may require data to be restricted.
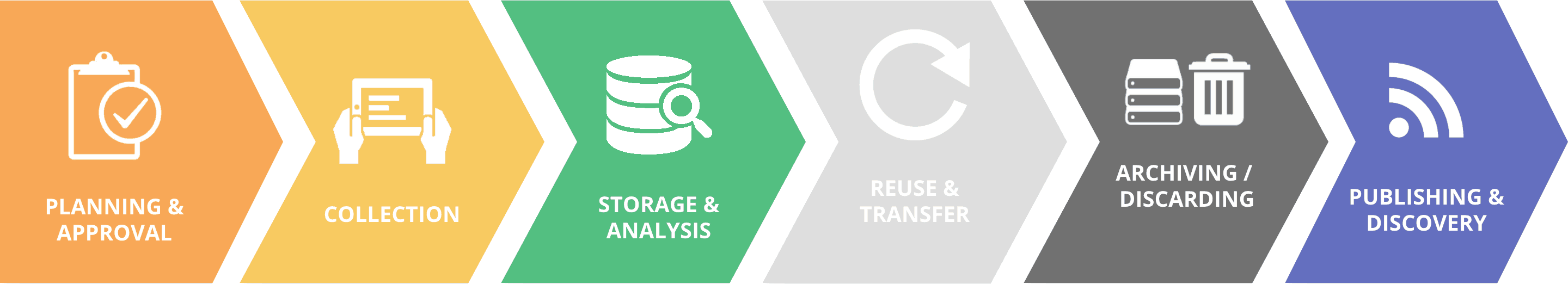
As an integral component of its advocacy for open data, the Platform promotes responsible data management through the entire research data lifecycle from planning, collecting, storing, disclosing or publishing, transferring, discovery and archiving.
These guidelines were created from information collected from: review on best and emerging practices across various sectors in the fast changing landscape of privacy and ethics (130 external resources); privacy and ethic materials sourced from seven CGIAR centers; first draft was circulated for input and feedback across CGIAR and incorporated into this edition. It’s important to note that this is an evolving document, the next stage is to consult externally for further input.
These Guidelines are intended to assist agricultural researchers handle privacy and personally identifiable information (PII) in the research project data lifecycle.

Ensure compatibility with the DMP-PII (as above) and also the purpose for which prior informed consent has been obtained
Ensure PII is stored securely to protect privacy, through organizational or project specific safeguards to prevent unauthorized access, accidental disclosure or breach of data (physical & technical)
Don’t store data in unsecured locations or on unsecured devices or servers
Don’t store encrypted data and encryption keys in locations where they can be easily accessed simultaneously
Don’t underestimate the importance and value of administrative safeguards to standardize practices (i.e. organizational policies, procedures and maintenance of security measures that are designed to protect private information, data and access)